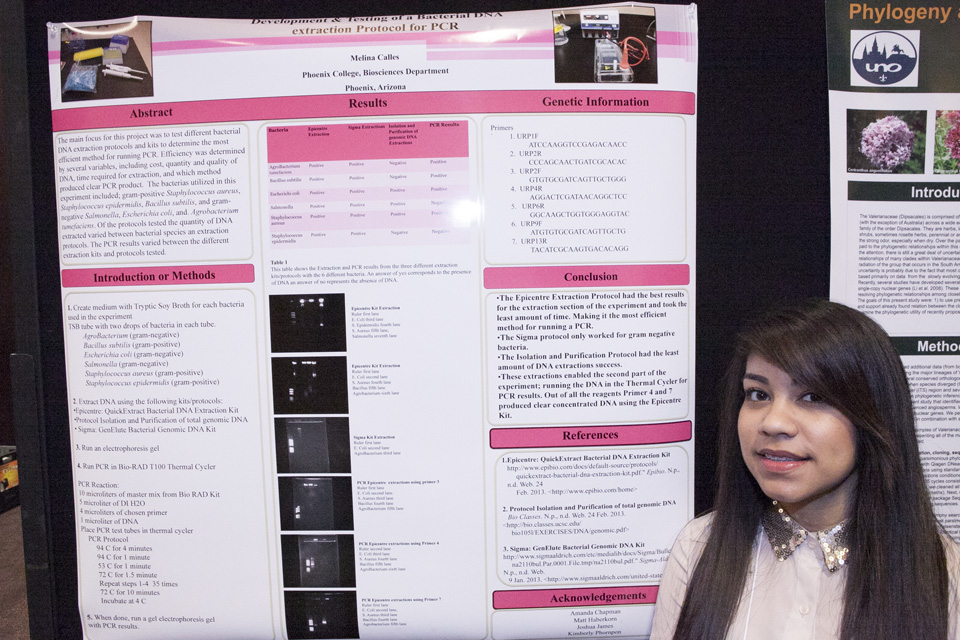
This week has been a bit crazy because of how little time we
have left in the semester and how much there is still to get done. I am glad to
say that even though the Estrella Conference is right around the corner (next
Thursday) I do not feel too stressed about it, I actually feel pretty calm.
That is my priority as of right now though, I need to get my poster draft done
by tomorrow, and have it ready to go for Monday. I am still waiting on the few results I hope
to include in my poster that were done this week.
For this week I noticed that I had two results of the RFLP
technique using the same enzyme (Ecor1), so I decided to try two different enzymes
and compare them both using the same type of bacteria. The enzyme I used was
Hind III and it had its corresponding buffer. I have provided the protocol for
the RFLP I used with each enzyme. Just
to clarify the DNA from the bacteria I used were again extracted from a 24 hour
culture and seemed to have worked great! I wanted to try all seven bacteria for
both protocols even though I knew that I was not successful with a few
extractions (3-4 bacteria did not work) because I have learned that it is sometimes
hard to notice bands using the ultraviolet light because of the concentration
of the sybr green. Below are the results. |
| Hind III enzyme |
Bacteria :
RFLP Protocol
- 10 ul buffer
- 86 ul DNA
- 4 ul of Ecor1 enzyme or Hind III enzyme
- Incubated at 37 c degrees for 1 hour
I also decided to run another PCR RAPD protocol on the same
extractions. For the PCR technique I used the DNA which had the best
extractions and used primers 3, 4 and 6. I thought after a few negative results
using primer 7 there would be no use trying it again. Since I ran out of
primers I had to dilute the ones we had in stock. Below I have the calculations
I made for this.
Bacteria:
- S. marcescens
- S. enterica
- B. subtilis
- S. epidermidis
- E. coli
- S. aureus
- P. stuartii
Primers
- 2 ul primer 3 + 38 ul TE
- 2ul primer 4 + 38 ul TE
- 2 ul primer 6 + 38 ul TE
PCR Protocol
- 10 ul master mix
- 5 ul DI H2O
- 4 ul primer (3, 4 or 6)
- 1 ul DNA